
How To Test Electric Conductivity Using Water Electric Conductivity
Enhancing the electrical conductivity of liquid crystal (LC) circumvents challenges for application in advanced electronic components. Toward this, using additives made of different nanostructures.

Conductivity of Electricity in different Liquids YouTube
The electrical conductivity is calculated from the change in measured conductance with immersion. This technique was validated in 1.0, 0.1, and 0.01 D KCl ~aq solutions at room temperature. Measured ! electrical conductivities were within 60.5% of the standard reference values. 1998 American Institute of Physics. S0034-6748 98 04909-0 @ ~ ! #

Electrical conductivity of liquids for class 8 (branch 2) YouTube
Ionic liquids (ILs) are organic salts composed mainly of anions and cations with melting temperatures below 100 °C. They are characterized by unique properties, including non-volatility, non-inflammability, wide electrochemical windows, high thermal stability, and good electrical conductivity (Wagner et al. 2020).The properties of ILs can be tuned by selecting the appropriate combination of.

Calculated fluid electrical conductivity (EC) of individual inflow
Electrical conductivity is the reciprocal quantity of resistivity. Conductivity is a measure of how well a material conducts an electric current. Electric conductivity may be represented by the Greek letter σ (sigma), κ (kappa), or γ (gamma). Table of Resistivity and Conductivity at 20°C Factors That Affect Electrical Conductivity

Electrical Conductivity of Liquids YouTube
Gao et al. have suggested two different lubricant formulations to be used in EVs and HEVs: (1) a high conductivity lubricant (3 × 1 0 − 5 to 2 × 1 0 − 4 µS cm-1 at room temperature) for minimizing electrical charge buildup and protecting against spark-related wear in high voltage components [21]; and (2) a low conductivity lubricant (5 × 1 0 − 7 to 3 × 1 0 − 5 µS cm-1 at room.

Electrical conductivity Stock Image A250/0126 Science Photo Library
The electroconductivity is a physical quantity for describing the degree to which a specified material conducts electricity. It is calculated as the ratio of the current density in the material to the electric field which causes the flow of current. It is the reciprocal of the resistivity [ 20 ].

Electrical conductivity of liquids class 8 questions and answers Az
Keywords: electrical conductivity, cell constant, sources of measurement error, electrode polarization, four-electrode method 1. INTRODUCTION Among the liquids conducting electric current a great majority are electrolyte solutions that are also the main component of all living organisms. Hence measurements of electrolytic conductivity.

ELECTRICAL CONDUCTIVITY OF LIQUIDS YouTube
Microsoft Word - Conductivity Chart of Liquids Carbon Tetrachloride Carboxylic Acid Chlorine Chloriacetic Acid M-Chloroaniline Chloroform Chlorohydrin Chocolate Liquor Coca Cola Syrup Coffee Extract Corn Syrup Cranberries Chrushed Cream Cheese Mix M-Creosol Cupric Chloride Cupric Nitrate Cupric Sulfate

LIQUID CONDUCTIVITY CELL model ELC 1.2 ANKO
Free Shipping on eBay

Testing the electric conductivity of liquids in Electrical Conductivity
The electrical conductivity of a liquid depends upon the number of ions per unit volume and upon their drift velocity. The drift velocity of an ion varies with the electric field intensity, with the mass of the ion, and with other factors as well. Thus the electrical conductivity of different liquids may be expected to have widely different values.

Conduction of Electricity in Liquids Electrolysis, Reduction at
Conductivity is one of the key physical properties influencing the performance of an electrolyte in such applications. In this study, an extensive database for conductivity of ionic liquids was compiled, containing around 3800 data points over the temperature range from 234 K to 484 K, from 134 previously published literature sources.
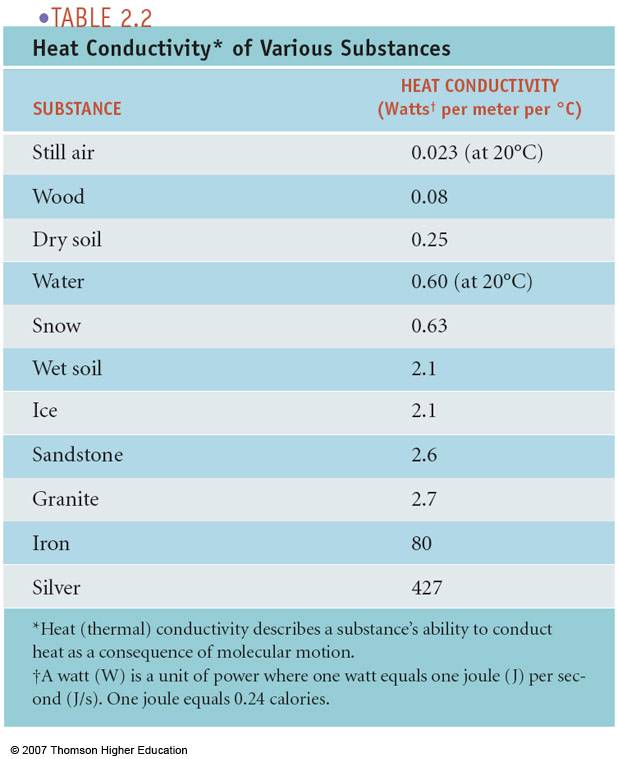
【ルすること】 Heat Transfer in Materials Forming Processes 9781848210523心の
Electrical conductivity (or specific conductance) is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter σ ( sigma ), but κ ( kappa) (especially in electrical engineering) and γ ( gamma) are sometimes used.

Electrical conductivity of liquids Big Chemical Encyclopedia
The electrical conductivity of ionic liquids at a specific concentration was measured using a DDS-307A conductivity meter at 25 °C. The temperature of the system was controlled within ± 0.01 °C.

Conduction of Electricity in Liquids Electrolysis, Reduction at
The Electrical Conductivity of Ionic Liquids: Numerical and Analytical Machine Learning Approaches by Theodoros E. Karakasidis , Filippos Sofos * and Christos Tsonos Condensed Matter Physics Laboratory, Department of Physics, University of Thessaly, 35100 Lamia, Greece * Author to whom correspondence should be addressed.
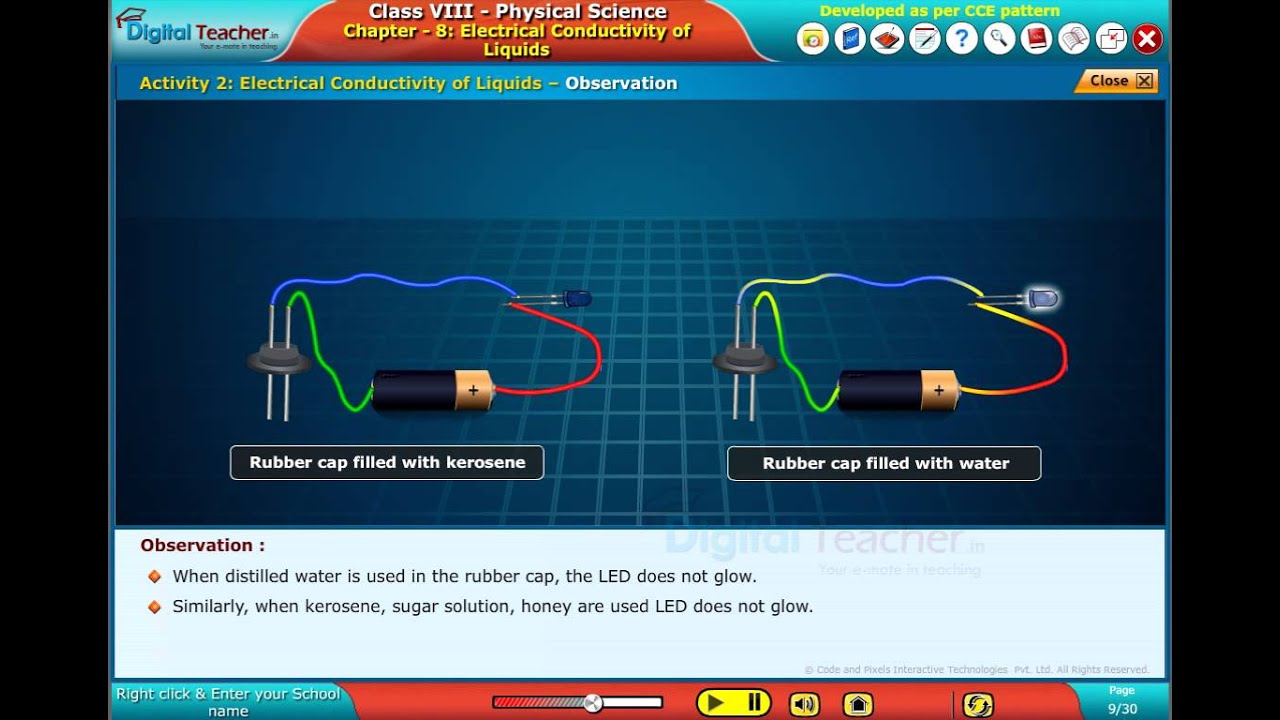
Electrical Conductivity of Liquids, Class 8 Physics Digital Teacher
Objectives. To observe electrical conductivity of substances in various aqueous solutions. To determine of the solution is a strong or weak electrolyte. To interpret a chemical reaction by observing aqueous solution conductivity. Electrical conductivity is based on the flow of electrons. Metals are good conductors of electricity because they.
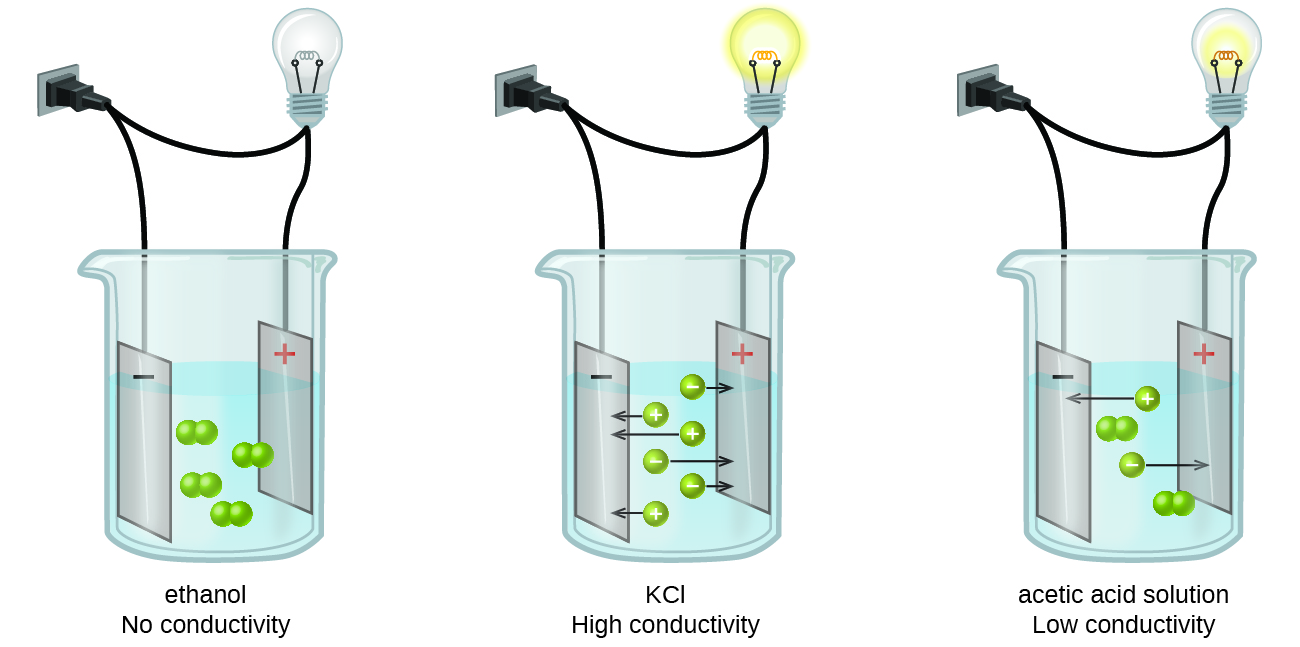
Each of the following reactions shows a solute dissolved in water
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter (S/m).